Biopharmaceuticals Market Size & Share - Growth Forecast Report (2026-2035)
Industry Insight by Product Type (Monoclonal Antibodies, Vaccines, Recombinant Proteins, Cell & Gene Therapies), by Therapeutic Application (Oncology, Autoimmune & Inflammatory Disorders, Infectious Diseases, Rare & Metabolic Disorders), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, Specialty Clinics), by Technology Platform (Recombinant DNA Technology, Monoclonal Antibody Engineering, Cell-Based Platforms, Gene-Editing Technologies), by Region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa)
| Status : Published | Published On : Jan, 2026 | Report Code : VRHC1306 | Industry : Healthcare | Available Format :

|
Page : 200 |
Biopharmaceuticals Market Size & Share - Growth Forecast Report (2026-2035)
Industry Insight by Product Type (Monoclonal Antibodies, Vaccines, Recombinant Proteins, Cell & Gene Therapies), by Therapeutic Application (Oncology, Autoimmune & Inflammatory Disorders, Infectious Diseases, Rare & Metabolic Disorders), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, Specialty Clinics), by Technology Platform (Recombinant DNA Technology, Monoclonal Antibody Engineering, Cell-Based Platforms, Gene-Editing Technologies), by Region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa)
Biopharmaceuticals Market Overview
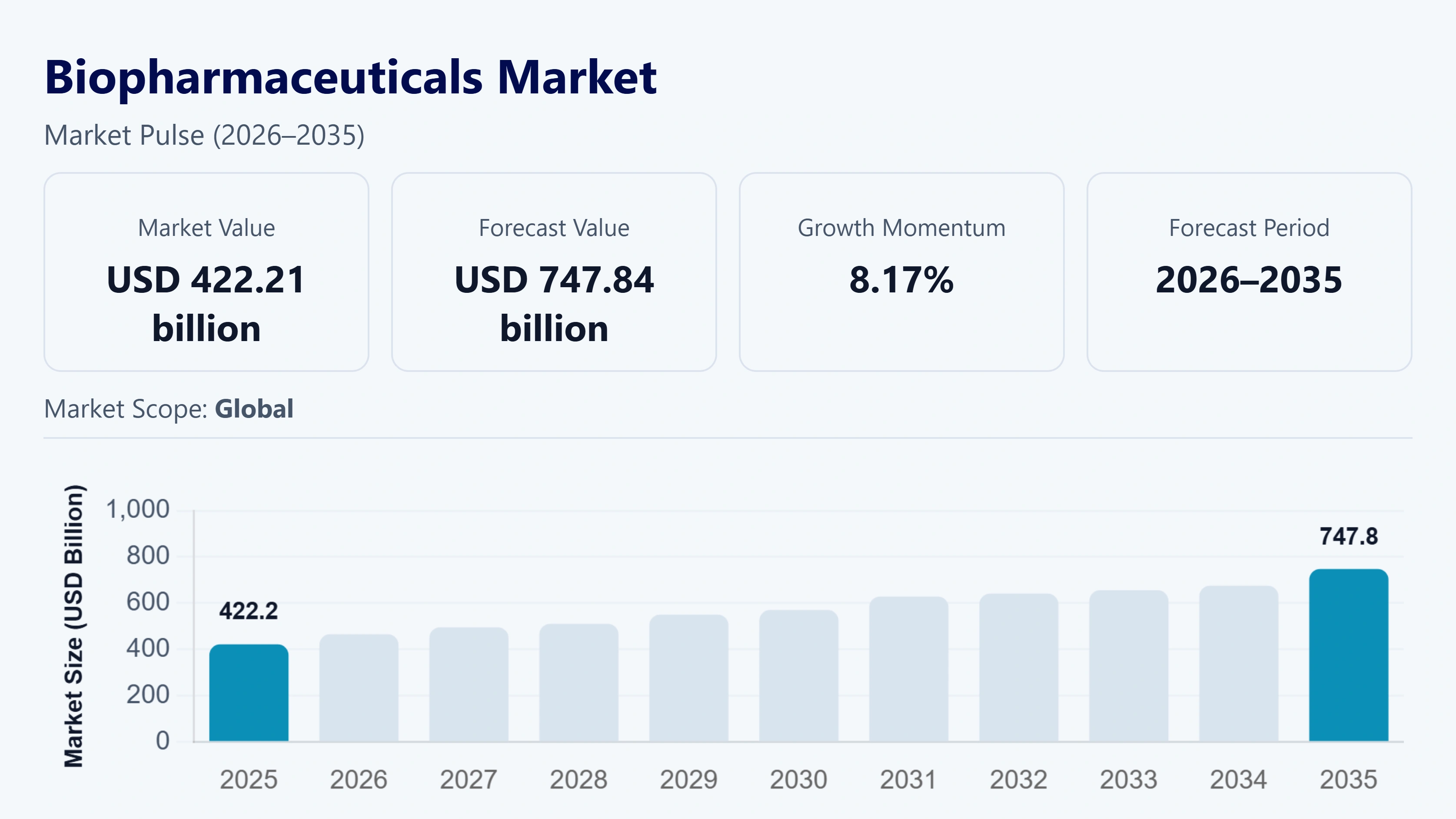
The global biopharmaceuticals market was valued at approximately USD 422.21 billion in 2025 and estimated to rise USD 465.86 in 2026, is projected to reach around USD 747.84 billion by 2035, growing at a CAGR of about 8.17 % from 2026 to 2035. This large-scale expansion is a direct reflection of the increasing global demand for cutting-edge biologic therapies in various fields of diseases such as oncology and immunology.
Biopharmaceuticals industry is influenced by major growth drivers. The rising prevalence of chronic and rare diseases is the major factor for the increased demand of biologic therapies. The progress in biomanufacturing technologies combined with substantial R&D investments provide a way for faster drug development and commercialization which in turn leads to more access globally.
Higher number of regulatory approvals for novel biologics and biosimilars have been good for market confidence. Moreover, the use of personalized medicine approaches and the expansion of healthcare infrastructure in the developing countries are the two factors which will result in the biopharmaceuticals market keeping its direction of growth for the next ten years.
Biopharmaceuticals Industry Dynamics
Market Trends
A major trend that is influencing the biopharma market is the fast incorporation of technologically advanced manufacturing systems like continuous bioprocessing and single-use technologies. With these methods, producers can reduce the time for the production of a product, scale up their enterprises more efficiently and be able to control the cost even under periods when the industry is plagued by cost-related problems. Manufacturers have raised their bets on flexible platforms that not only meet the supply demands of the clinical stage but also those of the commercial stage as therapies get more and more complex.
Furthermore, a transition greatly impacting the biopharma industry is the move toward next-generation biologics, such as cell and gene therapies, antibody-drug conjugates, and RNA-based treatments. These products give the very targeted therapeutic results, thus attracting heavy investments, both from the already existing companies and the new ones. The short clinical trials and regulatory support for these products are the main reasons for the fast innovations in this area and this trend is not limited to biopharma but applies to the whole healthcare industry.
Growth Drivers
The most significant growth driver for the biopharmaceutical sector is still the rise in chronic and rare diseases that are becoming more and more common. For example, diseases like cancer, autoimmune disorders, and metabolic diseases call for the use of drugs that are often not yet available in the market. The insufficiency of these new biologics means a continuous push resulting in the increasing demand of biologics with better efficacy and less side effects of long-term use.
The developing healthcare infrastructure, especially, in the less developed regions through which the market has been expanding is yet another reason why the demand for biopharmaceutical products is growing. Improved diagnostic capacities, more extensive insurance coverage, and fewer governments charging that sufficient funds be allocated to advanced therapeutics are all factors that contribute to the wider patient access to biopharma drugs. So, with the rising consciousness and maturing of the distribution networks, there is a pretty good chance of demand for biologics to increase in different regions.
Market Restraints / Challenges
The steep price of making biological drugs due to the complex process, stringent safety rules and use of living cells deter several smaller companies from accessing cutting-edge treatments.
Another barrier is the tough rules of approval of new drugs which delays the process due to lot of trial data, variable standards between countries, delaying plans for worldwide rollouts. These obstacles don’t just raise the chance of failure but also cost more money while holding back medicines from reaching people sooner, particularly where approvals drag on.
Supply chains often struggle, especially in this field as biologics need strict conditions, careful transport, while relying on steady refrigerated delivery systems. Problems like missing materials, political tensions, or shipping hiccups might shake output consistency. These weak spots mean ongoing challenges firms have to handle just to keep items accessible.
When patents run out, drug makers lose their edge on popular meds. That opens doors for copy versions, which pushes prices down. Even though more patients can get treatments, original creators see profits shrink.
Market Opportunities
Growing funding in personalized healthcare gives biopharma a solid chance. Since gene and cell analysis is spreading, people want more custom biological treatments fitted to unique care plans. Because of this change, scientists dig further into focused approaches while new treatments emerge - boosting results.
Emerging markets hold big growth opportunities down the road. Thanks to sharper diagnostics, growing health spending, or strong policy backing, biologics are gaining more ground. Firms moving in early might tap into gaps in care, larger groups of patients, or better supply networks - each helping new treatments spread further.
Technology opens new doors for better performance and expansion. Because of smart algorithms, rapid testing methods, or upgraded bio-production systems, firms can spot strong contenders quicker while cutting costs on biological drugs. As a result, these upgrades shorten research phases, lower manufacturing hurdles, also boost market edge in multiple treatment areas.
Working together - between universities, biotech startups, or big drug makers - is now more crucial than before. Teams join forces to spread out risks while pooling unique skills that speed up initial research phases. Instead of going it alone, groups link up so they can push tough biological projects forward. Because of this teamwork, fresh medical treatments reach worldwide patients quicker than in the past.
Global Biopharmaceuticals Market Report Coverage
|
Report Metric |
Details |
|
Historical Period |
2020 - 2024 |
|
Base Year Considered |
2025 |
|
Forecast Period |
2026 - 2035 |
|
Market Size in 2025 |
USD 422.21 Billion |
|
Revenue Forecast in 2035 |
USD 747.84 Billion |
|
Growth Rate |
8.17% |
|
Segments Covered in the Report |
Product Type, Therapeutic Application, Distribution Channel, Technology Platform |
|
Report Scope |
Market Trends, Drivers, and Restraints; Revenue Estimation and Forecast; Segmentation Analysis; Companies’ Strategic Developments; Market Share Analysis of Key Players; Company Profiling |
|
Regions Covered in the Report |
North America, Europe, Asia Pacific |
|
Key Companies |
|
|
Customization |
|
Biopharmaceuticals Market Segmentation
By Product Type
Monoclonal antibodies are the major players in the biopharmaceuticals market that account for almost 40% of the total revenue because of their wide usage in oncology and autoimmune treatments. Vaccines have around 22%, recombinant proteins 20%, and cell and gene therapies 10–12%, which show the progress of innovations and the emerging adoption of these products although the market shares are small.
The increasing rate of chronic diseases is the main factor that necessitates the use of monoclonal antibodies, as doctors are increasingly applying targeted therapies to manage diseases such as cancer and autoimmune disorders. This necessity will probably extend even further as biologics prove to be more effective and safer than the conventional therapies.
New technologies in the production of vaccines and recombinant proteins make the production process efficient and scalable. The use of single-use systems and continuous bioprocessing helps to cut down the production costs and improve the quality of the products, thus allowing speedy commercialization and making the products accessible to more patients from different therapy areas.
Regulatory support and incentives for innovative treatments, mainly cell and gene-based products, are the key factors that fuel investments in R&D. Accelerated approval pathways and orphan drug designations that shorten the time taken to reach the market thus giving the companies a chance to take advantage of the latest demand and be able to keep their growth momentum across varied product portfolios are among the other factors.
By Therapeutic Application
Biopharmaceuticals market is dominated by oncology which is estimated to have a share of about 33%. This is followed by autoimmune and inflammatory disorders with a share of around 25%, infectious diseases 16%, and rare/metabolic disorders 12–14%. These numbers show the most significant contribution of oncology to the revenues while other segments act as steady sources of growth.
Oncology is the major source of the increase in biologics' pipeline activities and their approvals, thus offering high specificity and improved clinical outcomes. The growing prevalence of cancer worldwide pushes the demand for targeted therapies keeping the market demand at a high level.
Autoimmune and inflammatory disorders are the source of the growth potential as new biologics prove to be effective and safe in the long-term. Through tumor awareness and early diagnosis, more patients become able to get these treatments especially in developed and emerging markets.
Treatment of infectious and rare diseases is the main reason for the industry to expand by the introduction of new vaccines, antivirals, and orphan biologics. The progress in personalized medicine open-ups tailor-made interventions thus creating small yet profitable markets and also encouraging the inflow of money to specialized research and development.
By Distribution Channel
Hospital pharmacies are at the forefront of the biopharmaceuticals market and thus have a revenue share that is estimated at around 42–45%. Moreover, the retail pharmacies have a share of about 30% while the online pharmacies and the direct-to-patient services have 15% each. The rest of the channels serve the niche segments and thus facilitate additional market penetration.
Expansion of hospital pharmacy is due to the institutional adoption of biologics that are also the guarantee for the proper storage, preparation, and patient monitoring. Most of the complicated therapies need professionals to supervise them thus enhancing the demand through hospital networks.
Retail pharmacies are in a favorable position to take advantage of the increasing outpatient use of biologics and in doing so they give freedom of choice and scalability to customers. The growth of the chronic disease management programs is the main driver that will continue to support the retail pharmacy sector in terms of market expansion.
Among others, the digital channels such as online pharmacies and direct-to-patient services are the future growth sources that have already started to emerge. Patients' preference for home delivery, adherence monitoring, and telemedicine integration are factors that lead to the wide use of these means, especially among people living in urban areas and who are tech-savvy.
By Technology Platform
The majority of the biopharmaceuticals market (about 38%) is based on recombinant DNA technologies with monoclonal antibody engineering coming second (30%), cell-based therapies (12%), gene-editing (10%), and the rest of the emerging platforms (8%) making up the rest of the market. This spread shows that the conventional platforms have a majority of the market, while there is also growth potential for the innovative therapies.
Recombinant DNA technologies are the main factors behind the increase in the market through their features such as efficiency, scalability, and demonstrated therapy areas efficacy. They enable a high volume of production and also make the easiest allow the broad adoption of biologics of a conventional nature.
The expansion of monoclonal antibody engineering in oncology and autoimmune diseases is the major cause that leads to the specification being highly specific and patient outcomes improved. The growth of the pipeline and the clinical adoption are the two main factors that strengthen the leadership position in the market.
They are going to be the major sources of growth in the near future; those platforms that use cells and gene-editing techniques. Increases in R&D expenditures due to regulatory support, successful clinical trials, and applications in personalized medicine R&D place these platforms in a future of rapid growth in highly specialized therapeutic areas.
Regional Insights
North America
The biopharmaceuticals market in North America is the biggest one worldwide, accounting roughly for 45–50% of the total market revenue. The pace of growth is mainly influenced by the region's considerable healthcare spending, a well-developed R&D research base, and the early use of biologics. The region is primarily led by oncology therapies and those for autoimmune disorders, which are, therefore, able to attract a full network of clinical trials and benefit from rapid regulatory approvals.
As a result of the rise in the prevalence of chronic diseases, especially cancer and autoimmune disorders, the market is expanding significantly. Patients want biologic therapies that are more targeted, and companies keep on investing in their pipeline. Insurance plans and reimbursement regulations have been put in place to facilitate access to these drugs, so that development may continue not only in hospital but also in retail pharmacy sectors.
Innovations in technology serve as a strong proper foundation for America to keep its regional leadership. The usage of the latest manufacturing methods, disposable bioprocessing, and AI-driven drug discovery all make the biologic production and its market entry much faster. What is more, the continuous partnership between universities, biotech startups, and big pharmaceutical companies is what eventually leads to first-stage innovation thereby strengthening the leadership of North America in global biologics creation and implementation.
New cell and gene therapies, as a result of regulatory incentives and orphan drug programs, are on their way and will soon become a significant part of the revenue basket in the region, which will confirm the existence of the market expansion in about next 5-7 years, as the trend of personalized medicine goes mainstream.
Asia Pacific
Biopharmaceuticals market in the Asia Pacific region is quickly growing and is responsible for around 18-22% of global revenue. Some of the major factors that are playing a supportive role in growth include the improvement in the healthcare infrastructure, increased healthcare expenditure, and a large number of biologics awareness programs in countries like China, India, and Japan. The major drive behind the demand for biologics is the increasing occurrence of chronic diseases.
The region is also witnessing an uptake of innovative therapies due to several measures taken by the government such as reimbursement schemes and facilitating the regulatory process. Mass production of the biotherapeutics can be made more cost-effective and more products can be made easily available through proper partnerships among local and foreign manufacturers, as well as by the investment in local R&D centers that may ultimately lead to better acceptance of monoclonal antibodies, vaccines, and recombinant proteins throughout the Asia Pacific region.
The participation of the private sector in various activities related to healthcare, the local companies building a good rapport with the multinationals, and the hospitals expanding their network are all giving a big push to the distribution sector. Besides this, outpatient and digital pharmacy channels are also thriving, therefore providing more users with easy access to their treatment in urban as well as in semi-urban areas, thus helping the Asia Pacific market sustain its upbeat pace.
The proliferation of biosimilars and novel biologics creating new openings for faster growth have become the company's strategy in leveraging the large patient population for clinical trials and rapid uptake, particularly in the fields of oncology and autoimmune therapies. Hence, there are some countries that are committing themselves to increasing investment in education and awareness programs which will ultimately lead to greater market penetration in these countries.
Europe
The biopharmaceuticals market in Europe constitutes a little more than one-fifth (around 20-24%) of the worldwide revenue, and it is mainly influenced by mature healthcare systems and strong medical R&D capabilities. The biologics for cancer and autoimmune will be the major contributors, assisted by the programs of early access, central regulatory frameworks and networks of distribution already established in Western Europe.
The prevalence of chronic diseases and the aging of the population increase the demand for biologic therapies. National policies on reimbursement, healthcare coverage together with the regulatory pathways that are very helpful for patient access are the factors that allow the different hospital as well as retail pharmacy channels to grow gradually.
Technological innovations, as well as the local manufacturing infrastructure, are in line with the growth and make the supply of biologics feasible for the companies while at the same time meeting high-quality standards. The collaboration between biotech companies and academia also speeds up the pipeline and clinical trials for new therapies.
Biosimilars are becoming major players in market dynamics, which is benefitting the process of making the products affordable and letting more patients access them. A combination of innovation, policy support, and patient awareness contributes to Europe being able to keep a steady and sustainable upward trajectory in the biopharmaceuticals market.
Competitive Landscape / Company Insights
Mini Profiles
Pfizer ranks among the top biopharmaceutical companies globally and possesses a varied product portfolio that extends to vaccines, oncology, and immunology products. The company operates worldwide with a solid presence and is eager to put half of the money from their research and development into biologics and innovative treatment. Besides, collaborations with academic institutions and biotech companies not only speed up new drug developments but also help Pfizer gain a competitive edge in the future market.
Roche is a giant Swiss biopharmaceutical company that mainly focuses on the development of oncology, immunology, and neuroscience biologics. The company promotes the use of precision medicine and supplement diagnostics to personalize patient treatment. Moreover, Roche has a strong pipeline of research and development in the areas of antibody-based and targeted therapies and shifts to global distribution networks to deliver biologics efficiently across the world.
Novartis is a diversified biopharmaceutical company that has a biologics portfolio mainly in oncology, immunology, and rare diseases. The company merges its internal R&D with external collaborations and purchase to improve its pipeline. Novartis is actively engaged in cell and gene therapies while still continuing with conventional biologics to help directly address the medical needs that are not yet met while ensuring future growth.
Biogen concentrates on biologics for the nervous system and immunology, and holds a commanding position in multiple sclerosis and other neurological diseases. The company has allocated resources to develop advanced biologic platforms and is on board with collaborative efforts with academic institutions to fast-track the development of the most cutting-edge treatments. Furthermore, strategic partnerships are a source of funding for new therapies and allow easy access to targeted therapy areas.
Amgen is a worldwide leader in biopharmaceutical industry with a large biologics-related portfolio in the fields of oncology, nephrology, and inflammation. The company main efforts are on monoclonal antibodies and novel biologics creation, which are kept healthy by strong R&D and well-equipped manufacturing facilities. Amgen is pulling out all the stops for efficient mass production to be able to satisfy global demand as it increases.
Recent Developments
Merck in the early part of 2025 made an announcement regarding an expansion in clinical trials of a next-generation monoclonal antibody targeting oncological conditions. The company also plans to bolster production capacity by investing heavily in manufacturing facilities to meet the anticipated global demand, and thus organically grow both their biologics production and their pipeline readiness.
Sanofi in mid-2025 unveiled an upgraded vaccine production plan, with a focus on better product yield and delivery efficiency. The program is geared to meet the growing immunization demands worldwide and is designed to facilitate the broader use of biologics in developing countries where the healthcare infrastructure is still in its infancy.
AstraZeneca announced a new partnership with a biotech company in March 2025, and the main goal is to jointly develop RNA-based therapies for rare diseases. By cutting down R&D timelines and bringing in novel treatment solutions, this partnership is targeted precisely at the need of the tiniest patient groups.
AbbVie broadened the scope of its biologics pipeline through the 2025 initiation of new clinical trials of the treatments for autoimmune and inflammatory disorders. The company likewise facilitated the manufacture of higher-volume production of monoclonal antibodies and complex biologics through the enhanced capabilities of the production sector.
At the middle of 2025, Novo Nordisk revealed their breakthrough next-generation biologics for diabetes and metabolic disorder. Besides, R&D and process innovation investments are supposed to uplift product efficacy and widen patient accessibility in major global markets.
Global Biopharmaceuticals Market Coverage
Product Type Insight and Forecast 2026 - 2035
- Monoclonal Antibodies
- Vaccines
- Recombinant Proteins
- Cell & Gene Therapies
Therapeutic Application Insight and Forecast 2026 - 2035
- Oncology
- Autoimmune & Inflammatory Disorders
- Infectious Diseases
- Rare & Metabolic Disorders
Distribution Channel Insight and Forecast 2026 - 2035
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Specialty Clinics
Technology Platform Insight and Forecast 2026 - 2035
- Recombinant DNA Technology
- Monoclonal Antibody Engineering
- Cell-Based Platforms
- Gene-Editing Technologies
Region Insight and Forecast 2026 - 2035
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Global Biopharmaceuticals Market by Region
- North America
- By Product Type
- By Therapeutic Application
- By Distribution Channel
- By Technology Platform
- By Region
- By Country - U.S., Canada, Mexico
- Europe
- By Product Type
- By Therapeutic Application
- By Distribution Channel
- By Technology Platform
- By Region
- By Country - Germany, U.K., France, Italy, Spain, Russia, Rest of Europe
- Asia-Pacific (APAC)
- By Product Type
- By Therapeutic Application
- By Distribution Channel
- By Technology Platform
- By Region
- By Country - China, Japan, India, South Korea, Vietnam, Thailand, Malaysia, Rest of Asia-Pacific
- Rest of the World (RoW)
- By Product Type
- By Therapeutic Application
- By Distribution Channel
- By Technology Platform
- By Region
- By Country - Brazil, Saudi Arabia, South Africa, U.A.E., Other Countries
Table of Contents for Biopharmaceuticals Market Report
1. Research Overview
1.1. The Report Offers
1.2. Market Coverage
1.2.1. By
Product Type
1.2.2. By
Therapeutic Application
1.2.3. By
Distribution Channel
1.2.4. By
Technology Platform
1.2.5. By
Region
1.3. Research Phases
1.4. Limitations
1.5. Market Methodology
1.5.1. Data Sources
1.5.1.1.
Primary Research
1.5.1.2.
Secondary Research
1.5.2. Methodology
1.5.2.1.
Data Exploration
1.5.2.2.
Forecast Parameters
1.5.2.3.
Data Validation
1.5.2.4.
Assumptions
1.5.3. Study Period & Data Reporting Unit
2. Executive Summary
3. Industry Overview
3.1. Industry Dynamics
3.1.1. Market Growth Drivers
3.1.2. Market Restraints
3.1.3. Key Market Trends
3.1.4. Major Opportunities
3.2. Industry Ecosystem
3.2.1. Porter’s Five Forces Analysis
3.2.2. Recent Development Analysis
3.2.3. Value Chain Analysis
3.3. Competitive Insight
3.3.1. Competitive Position of Industry
Players
3.3.2. Market Attractive Analysis
3.3.3. Market Share Analysis
4. Global Market Estimate and Forecast
4.1. Global Market Overview
4.2. Global Market Estimate and Forecast to 2035
5. Market Segmentation Estimate and Forecast
5.1. By Product Type
5.1.1. Monoclonal Antibodies
5.1.1.1. Market Definition
5.1.1.2. Market Estimation and Forecast to 2035
5.1.2. Vaccines
5.1.2.1. Market Definition
5.1.2.2. Market Estimation and Forecast to 2035
5.1.3. Recombinant Proteins
5.1.3.1. Market Definition
5.1.3.2. Market Estimation and Forecast to 2035
5.1.4. Cell & Gene Therapies
5.1.4.1. Market Definition
5.1.4.2. Market Estimation and Forecast to 2035
5.2. By Therapeutic Application
5.2.1. Oncology
5.2.1.1. Market Definition
5.2.1.2. Market Estimation and Forecast to 2035
5.2.2. Autoimmune & Inflammatory Disorders
5.2.2.1. Market Definition
5.2.2.2. Market Estimation and Forecast to 2035
5.2.3. Infectious Diseases
5.2.3.1. Market Definition
5.2.3.2. Market Estimation and Forecast to 2035
5.2.4. Rare & Metabolic Disorders
5.2.4.1. Market Definition
5.2.4.2. Market Estimation and Forecast to 2035
5.3. By Distribution Channel
5.3.1. Hospital Pharmacies
5.3.1.1. Market Definition
5.3.1.2. Market Estimation and Forecast to 2035
5.3.2. Retail Pharmacies
5.3.2.1. Market Definition
5.3.2.2. Market Estimation and Forecast to 2035
5.3.3. Online Pharmacies
5.3.3.1. Market Definition
5.3.3.2. Market Estimation and Forecast to 2035
5.3.4. Specialty Clinics
5.3.4.1. Market Definition
5.3.4.2. Market Estimation and Forecast to 2035
5.4. By Technology Platform
5.4.1. Recombinant DNA Technology
5.4.1.1. Market Definition
5.4.1.2. Market Estimation and Forecast to 2035
5.4.2. Monoclonal Antibody Engineering
5.4.2.1. Market Definition
5.4.2.2. Market Estimation and Forecast to 2035
5.4.3. Cell-Based Platforms
5.4.3.1. Market Definition
5.4.3.2. Market Estimation and Forecast to 2035
5.4.4. Gene-Editing Technologies
5.4.4.1. Market Definition
5.4.4.2. Market Estimation and Forecast to 2035
5.5. By Region
5.5.1. North America
5.5.1.1. Market Definition
5.5.1.2. Market Estimation and Forecast to 2035
5.5.2. Europe
5.5.2.1. Market Definition
5.5.2.2. Market Estimation and Forecast to 2035
5.5.3. Asia Pacific
5.5.3.1. Market Definition
5.5.3.2. Market Estimation and Forecast to 2035
5.5.4. Latin America
5.5.4.1. Market Definition
5.5.4.2. Market Estimation and Forecast to 2035
5.5.5. Middle East & Africa
5.5.5.1. Market Definition
5.5.5.2. Market Estimation and Forecast to 2035
6. North America Market Estimate and Forecast
6.1. By
Product Type
6.2. By
Therapeutic Application
6.3. By
Distribution Channel
6.4. By
Technology Platform
6.5. By
Region
6.5.1.
U.S. Market Estimate and Forecast
6.5.2.
Canada Market Estimate and Forecast
6.5.3.
Mexico Market Estimate and Forecast
7. Europe Market Estimate and Forecast
7.1. By
Product Type
7.2. By
Therapeutic Application
7.3. By
Distribution Channel
7.4. By
Technology Platform
7.5. By
Region
7.5.1.
Germany Market Estimate and Forecast
7.5.2.
U.K. Market Estimate and Forecast
7.5.3.
France Market Estimate and Forecast
7.5.4.
Italy Market Estimate and Forecast
7.5.5.
Spain Market Estimate and Forecast
7.5.6.
Russia Market Estimate and Forecast
7.5.7.
Rest of Europe Market Estimate and Forecast
8. Asia-Pacific (APAC) Market Estimate and Forecast
8.1. By
Product Type
8.2. By
Therapeutic Application
8.3. By
Distribution Channel
8.4. By
Technology Platform
8.5. By
Region
8.5.1.
China Market Estimate and Forecast
8.5.2.
Japan Market Estimate and Forecast
8.5.3.
India Market Estimate and Forecast
8.5.4.
South Korea Market Estimate and Forecast
8.5.5.
Vietnam Market Estimate and Forecast
8.5.6.
Thailand Market Estimate and Forecast
8.5.7.
Malaysia Market Estimate and Forecast
8.5.8.
Rest of Asia-Pacific Market Estimate and Forecast
9. Rest of the World (RoW) Market Estimate and Forecast
9.1. By
Product Type
9.2. By
Therapeutic Application
9.3. By
Distribution Channel
9.4. By
Technology Platform
9.5. By
Region
9.5.1.
Brazil Market Estimate and Forecast
9.5.2.
Saudi Arabia Market Estimate and Forecast
9.5.3.
South Africa Market Estimate and Forecast
9.5.4.
U.A.E. Market Estimate and Forecast
9.5.5.
Other Countries Market Estimate and Forecast
10. Company Profiles
10.1.
Pfizer
10.1.1.
Snapshot
10.1.2.
Overview
10.1.3.
Offerings
10.1.4.
Financial
Insight
10.1.5.
Recent
Developments
10.2.
Roche
10.2.1.
Snapshot
10.2.2.
Overview
10.2.3.
Offerings
10.2.4.
Financial
Insight
10.2.5.
Recent
Developments
10.3.
Novartis
10.3.1.
Snapshot
10.3.2.
Overview
10.3.3.
Offerings
10.3.4.
Financial
Insight
10.3.5.
Recent
Developments
10.4.
Biogen
10.4.1.
Snapshot
10.4.2.
Overview
10.4.3.
Offerings
10.4.4.
Financial
Insight
10.4.5.
Recent
Developments
10.5.
Amgen
10.5.1.
Snapshot
10.5.2.
Overview
10.5.3.
Offerings
10.5.4.
Financial
Insight
10.5.5.
Recent
Developments
10.6.
Merck
10.6.1.
Snapshot
10.6.2.
Overview
10.6.3.
Offerings
10.6.4.
Financial
Insight
10.6.5.
Recent
Developments
10.7.
Sanofi
10.7.1.
Snapshot
10.7.2.
Overview
10.7.3.
Offerings
10.7.4.
Financial
Insight
10.7.5.
Recent
Developments
10.8.
AstraZeneca
10.8.1.
Snapshot
10.8.2.
Overview
10.8.3.
Offerings
10.8.4.
Financial
Insight
10.8.5.
Recent
Developments
10.9.
AbbVie
10.9.1.
Snapshot
10.9.2.
Overview
10.9.3.
Offerings
10.9.4.
Financial
Insight
10.9.5.
Recent
Developments
10.10.
Novo Nordisk
10.10.1.
Snapshot
10.10.2.
Overview
10.10.3.
Offerings
10.10.4.
Financial
Insight
10.10.5.
Recent
Developments
11. Appendix
11.1. Exchange Rates
11.2. Abbreviations
Note: Financial insight and recent developments of different companies are subject to the availability of information in the secondary domain.
Frequently Asked Questions
Purchase Options
Latest Report
Research Methodology
- Desk Research / Pilot Interviews
- Build Market Size Model
- Research and Analysis
- Final Deliverable
Connect With Our Sales Team
- Toll-Free: +1-888-253-3960
- Phone: +91 9960 288 381
- Email: enquiry@vynzresearch.com
Biopharmaceuticals Market
